Need Assistance? Call us today! 602-478-9713
As many are aware, Inspire medical’s sleep apnea never stimulator was issued with a recall earlier this year.
FDA Definition: A situation in which there is a reasonable probability that the use of or exposure to a violative product will cause serious adverse health consequences or death.
The FDA has elevated a recall of Inspire Medical’s nerve-stimulating implant for the treatment of obstructive sleep apnea to Class I.
https://www.fda.gov/safety/industry-guidance-recalls/recalls-background-and-definitions
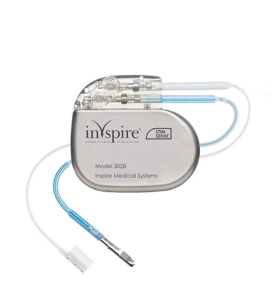
Here is an extract from a recent report in fiercepharma: The FDA issued a Class I recall for Inspire IV implantable pulse generators due to a manufacturing defect that could cause "electrical leakage," leading to fast battery drains, weakened therapy, or potential shocks. No injuries or deaths have been reported. The recall began on June 17, with healthcare providers and patients advised to schedule checkups. A noninvasive diagnostic process can identify the defect, but some devices may require revision surgery. The device, implanted near the collarbone, helps keep airways open during sleep by stimulating hypoglossal nerves. In June 2023, the FDA expanded the device's use to more patients, and by May, 1,250 U.S. medical centers were offering the therapy.
Then again the healthcare technology report informs that Inspire Medical Systems has secured FDA Approval for Next-Gen Neurostimulator as of early August.
Inspire Medical Systems Secures FDA Approval for Next-Gen Neurostimulator.
What are the negatives of Inspire sleep?
Besides surgical risks, some people may experience discomfort or unpleasant side effects from the Inspire sleep apnea device. These may be sensations like dry mouth, tongue discomfort, or feeling aware of the device.
Can Inspire sleep be removed?
It is possible to remove Inspire through a special surgical procedure.
Can you feel the Inspire device?
A patient can expect to feel a tingling sensation, or mild contraction in their tongue muscles.
What are the negatives with Inspire?
The most common side effects associated with Inspire treatment are tongue abrasion, mouth dryness, and discomfort stemming from the nerve stimulator. Some people experience muscle atrophy and partial tongue paralysis.
What will be the impact?
A recall can sometimes undermine providers' confidence in the safety and efficacy of the device. As a result, patients might explore other alternatives, such as oral appliance therapy (OAT) and positive airway pressure (PAP) therapies.
In response to the original question, "Will Inspire remain an innovative alternative to CPAP?" the outlook appears highly favorable for Inspire Medical Systems. The growing demand from patients who discontinue using CPAP and actively seek referrals for hypoglossal nerve stimulation surgery indicates strong, patient-driven interest in this alternative therapy.
Sources: FierceBiotech
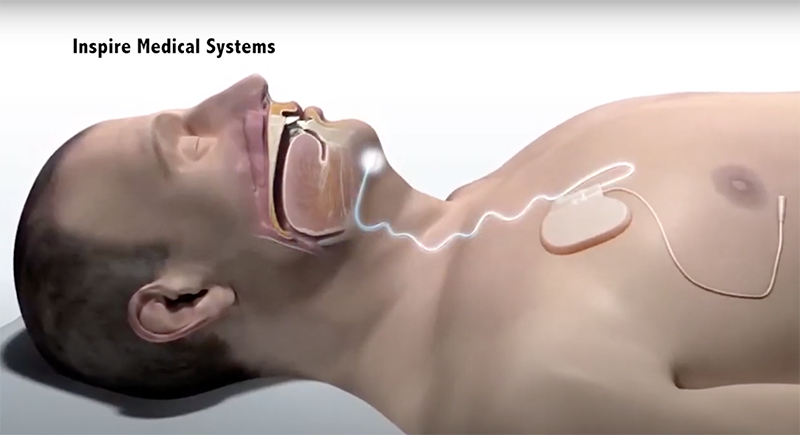
Image copyright Inspire Medical Systems